CORPORATE
What We Do
Biotek means the responsiveness and innovation of a small company, with the resources and market presence of a large company. Biotek offers a highly specialized and exclusive product range which extends through Sports
Medicine; Lower limb Osteotomy; Prostheses for Shoulder, Elbow and Radial Head; numerous kinds of instrumentation for arthroscopic and orthopedic procedures.
Biotek products are made of
- Bioabsorbable materials
- PEEK polymer (polyetheretherketone)-ASTM F2026
- Polymer fibers
- Pure titanium- ISO 5832-2/ASTM F67
- Titanium Alloy Ti-6Al-4V ELI (ISO 5832-3/ASTM F136)
- Stainless steel (ISO 5832-1 and ASTM F138/ASTM F139)
- Stainless steel (ASTM F899)
The materials are verified for biocompatibility, safety and risk management according to ISO 10993 and ISO 14971. The chemical composition, mechanical properties, physical properties and the purity of the microstructure meet the requirements specified by these standards. Appropriate laboratory material acceptance tests are carried out according to statistical criteria. The ‘material grade’ quality stands out for highest purity, complete lot traceability and constancy in manufacturing and processing. The quality of the implants is constantly proved and ensured by various in vitro and in vivo studies. Precision production techniques; surface treatments;
exact individual in-process and final inspections ensure the consistency in high quality standards of Biotek products in order to meet the strict regulations of EU directive (MDD 93/42/EEC). Biotek maintains a quality management system which is regularly certified in accordance with international standards ISO 13485 and MDSAP.
Biotek owes its rapid growth and international reach to its thorough expertise of the orthopaedics market and technologies. It employs highly experienced, innovation-driven professionals and engineers and manufactures more than 75,000-check? orthopedic instruments and implants a year.
Address
MD-04, Sanand-II GIDC,
Ahmedabad – 382 110, Gujarat, INDIA
Shoulder

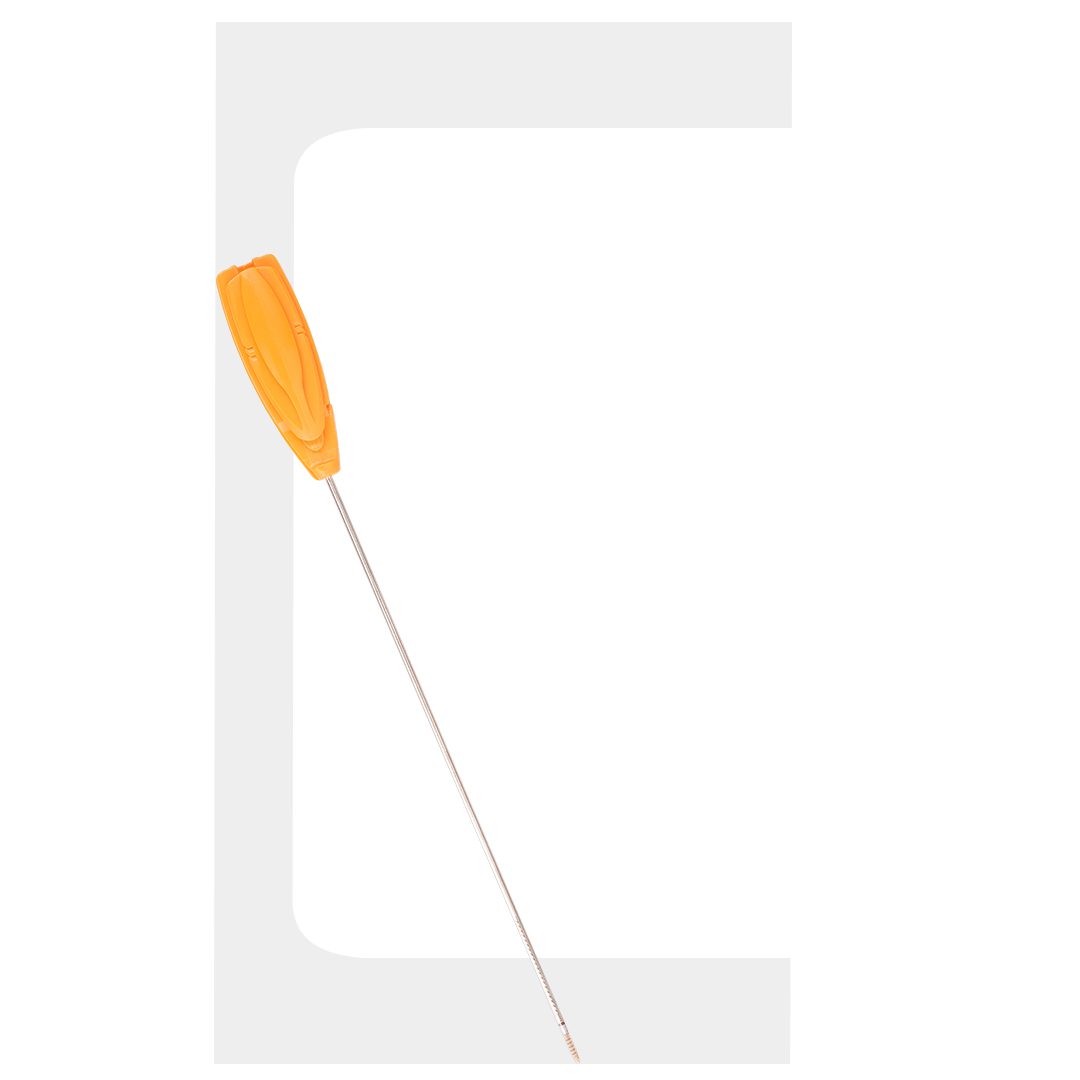
Fiberknot
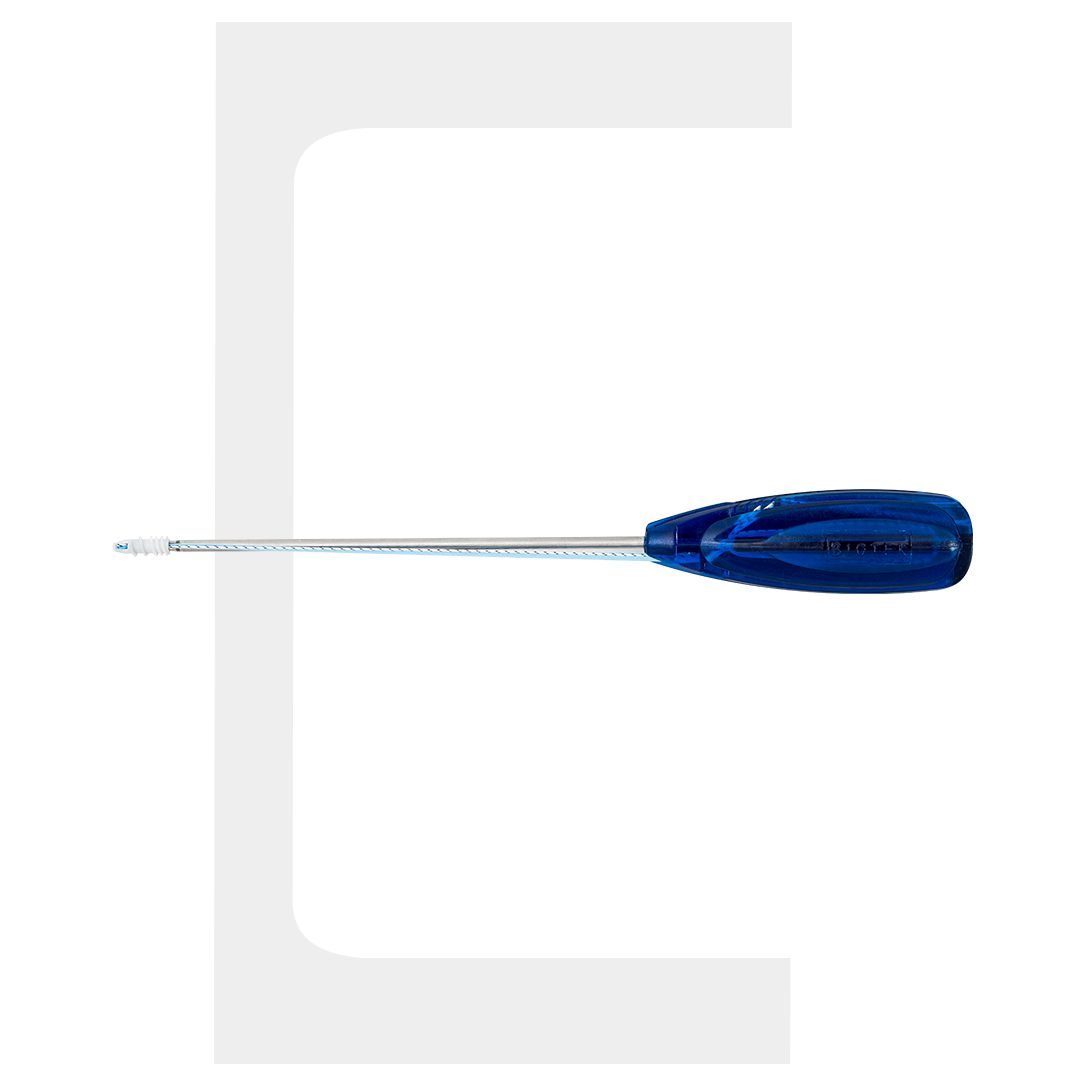
BIO-VIM Bioabsorbable Ligament Anchor

AC-FIX® Fixation button

VIMFIX®-BT Ligament Anchor
Osteotomy

Biofix-NP
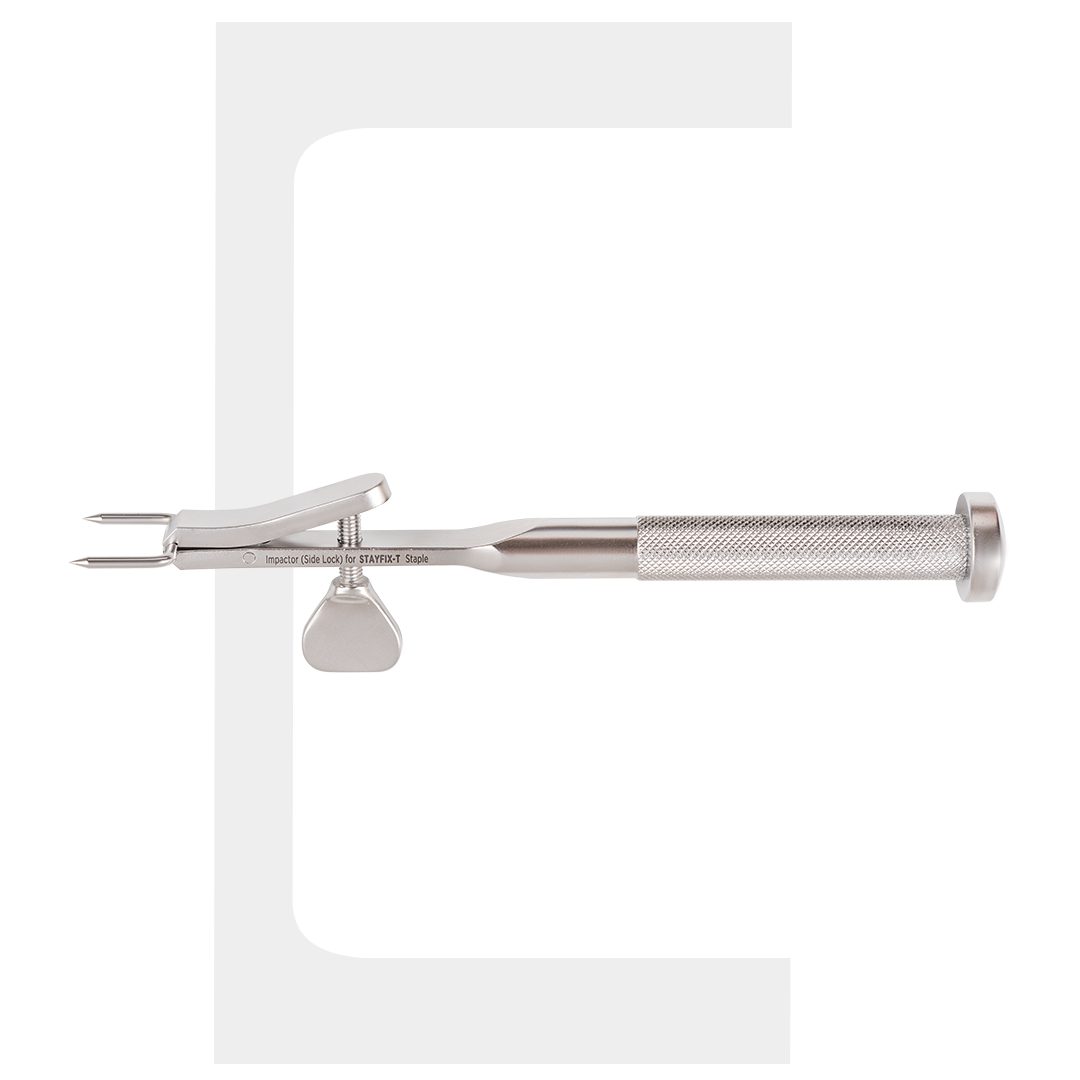
Stayfix-T

BioFix™-NP
Elbow
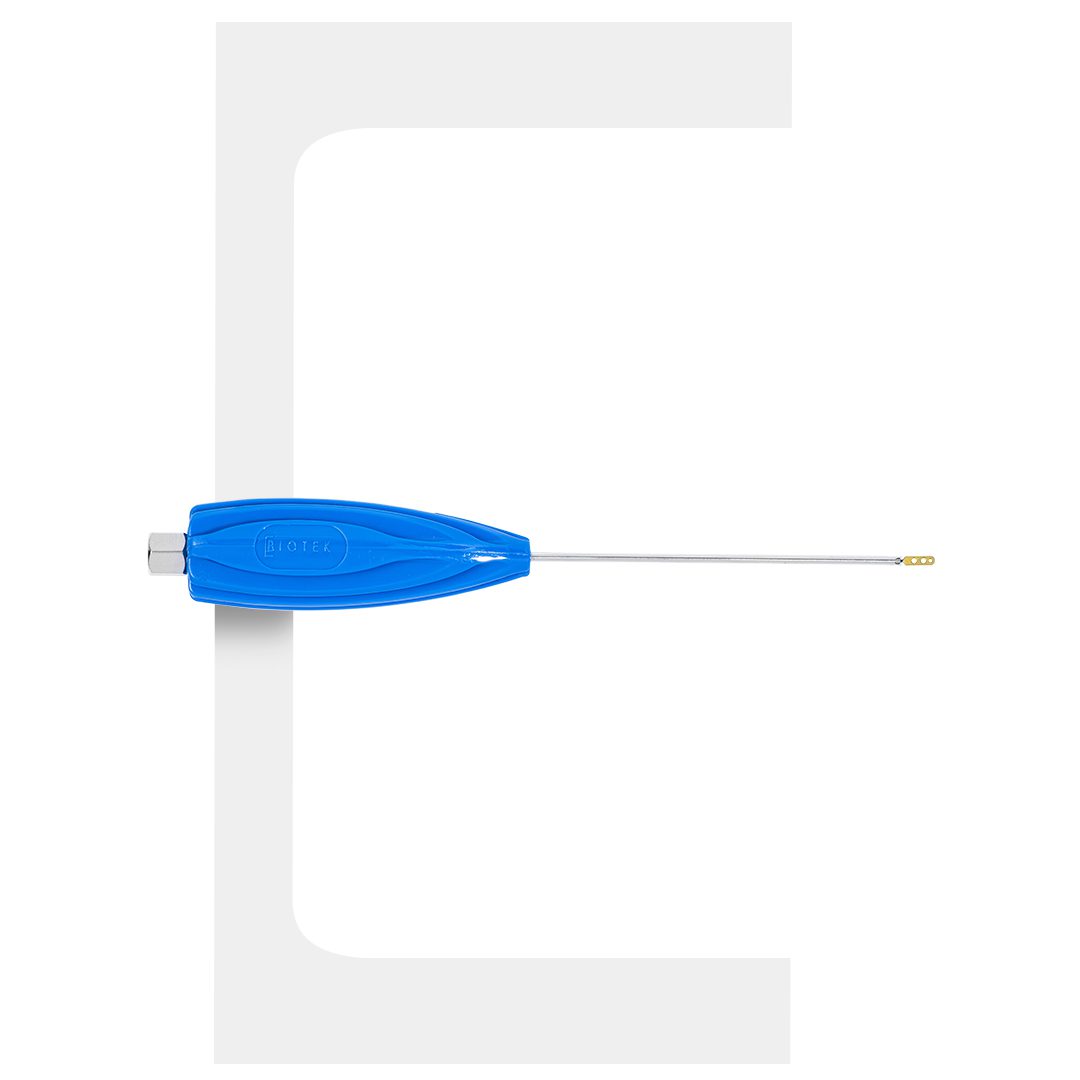
AK-FIX
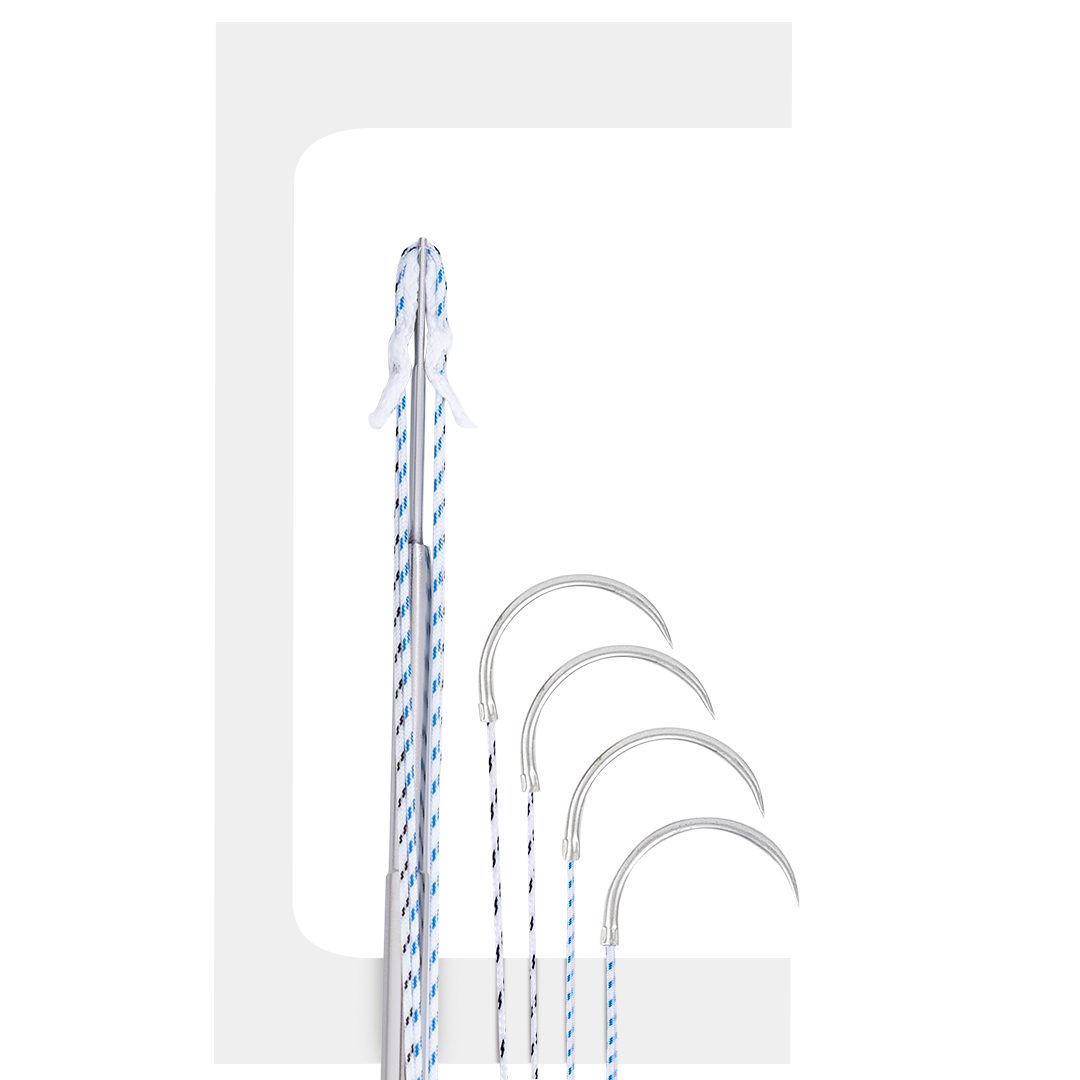
FIBERKNOT®

SOFTFIX-PK®

MINI-VIM PK®

FIBERKNOT®
Knee
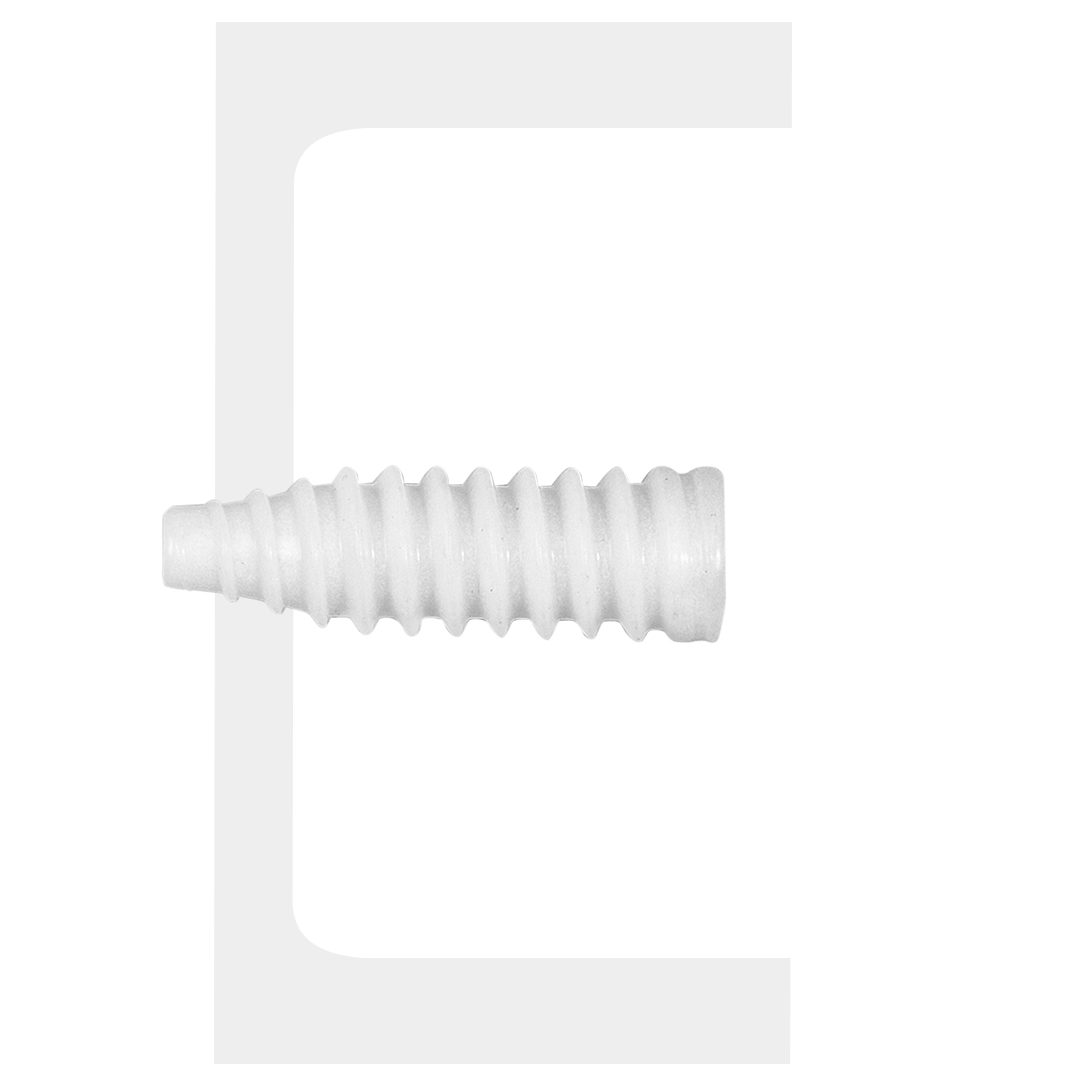
Biotwin
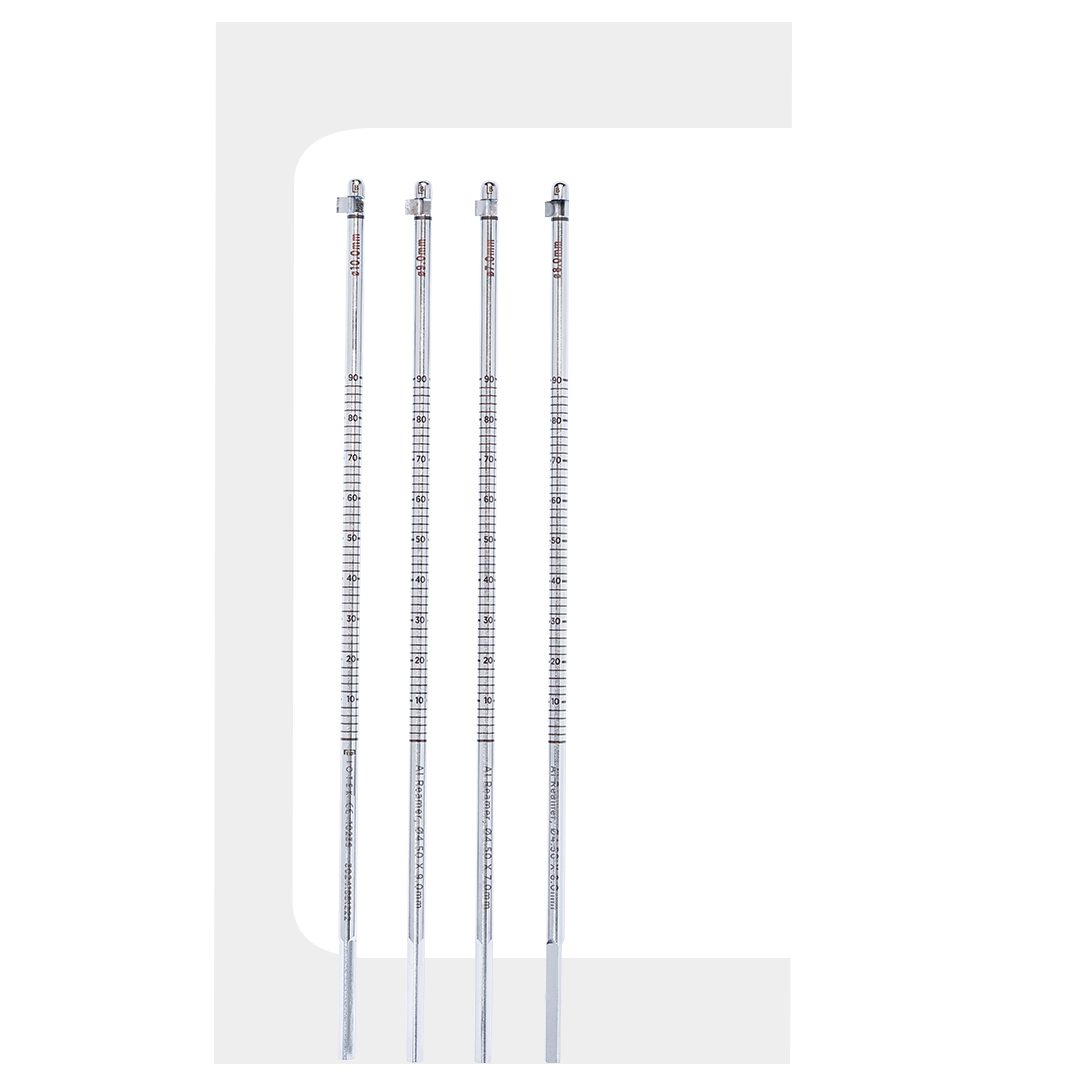
AI Reamer
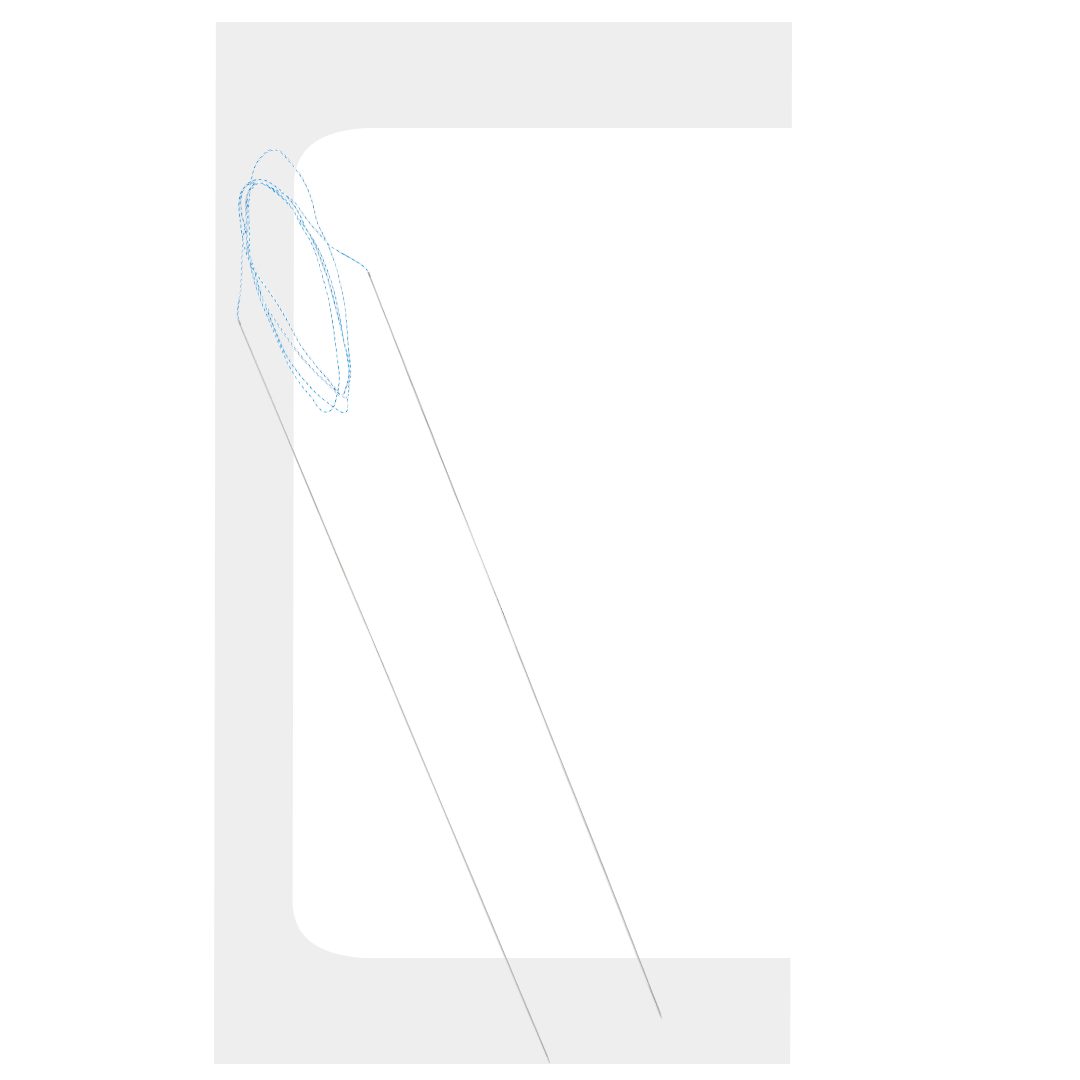
BIOCINCH®

Meniscus Repair Instruments
Foot & Ankle
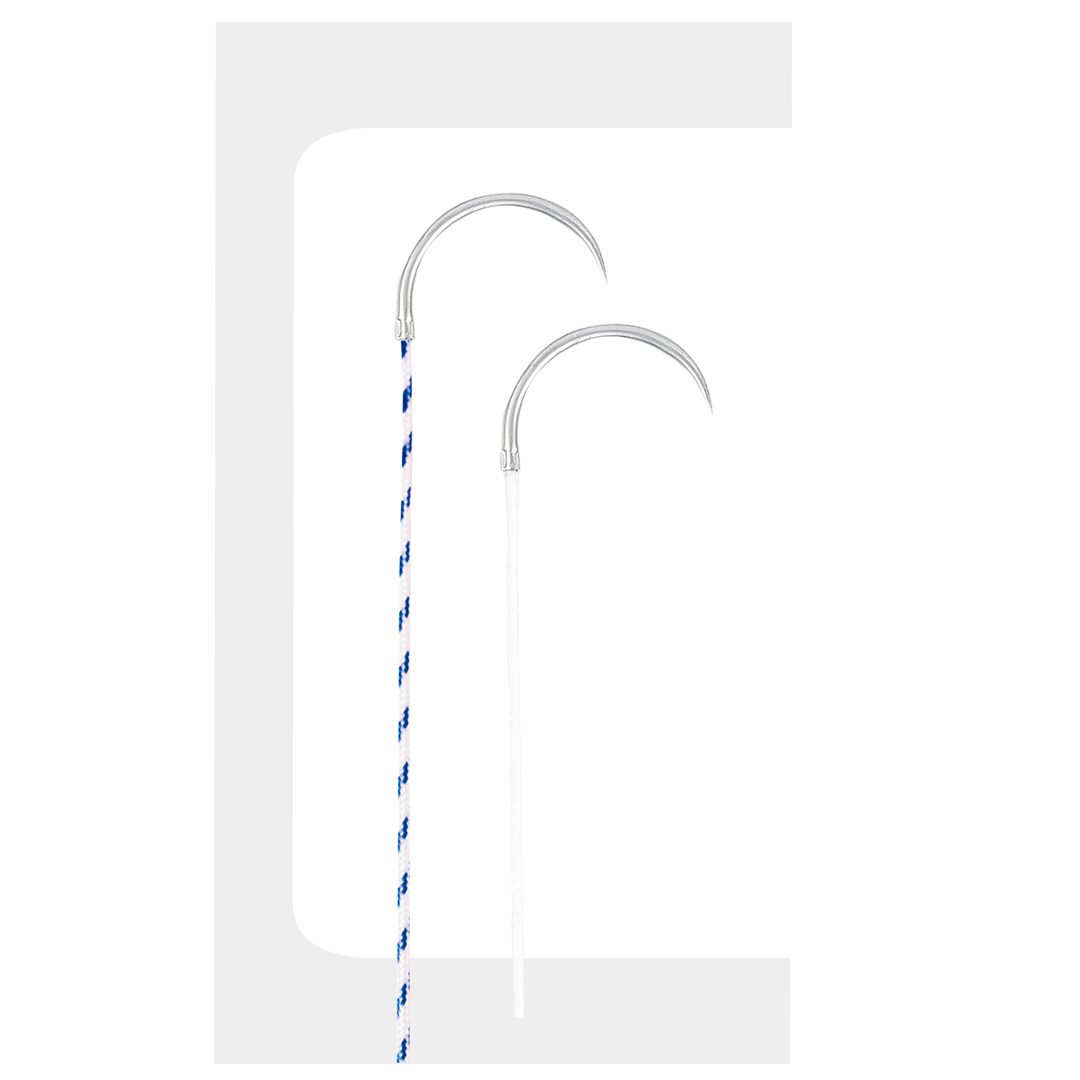
Biofiber

Interference Screws

FIBERKNOT®
Extremities

OSKAR

TEXX

URSA
Hand & Wrist
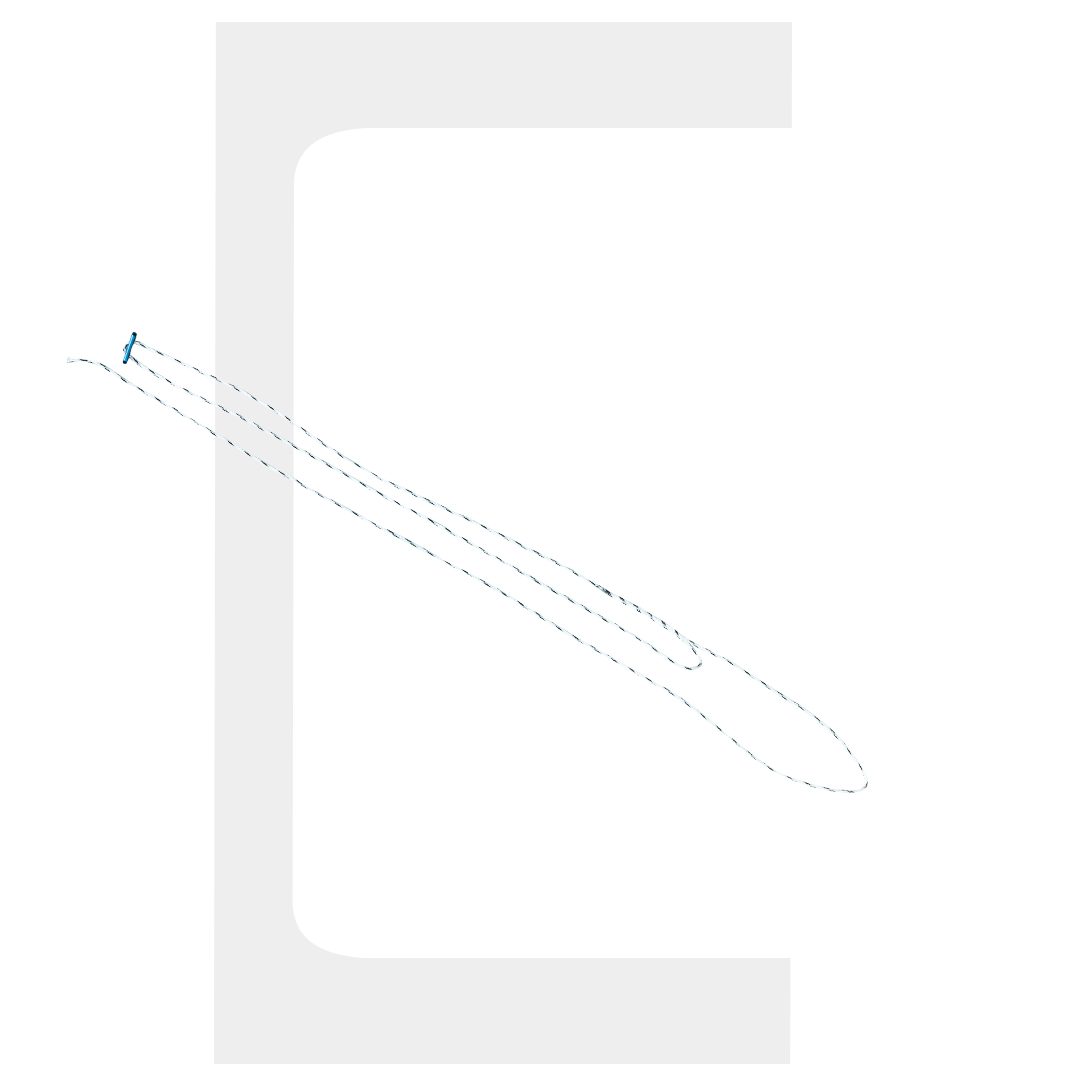
Buttonfix-W One
Fixation Buttons
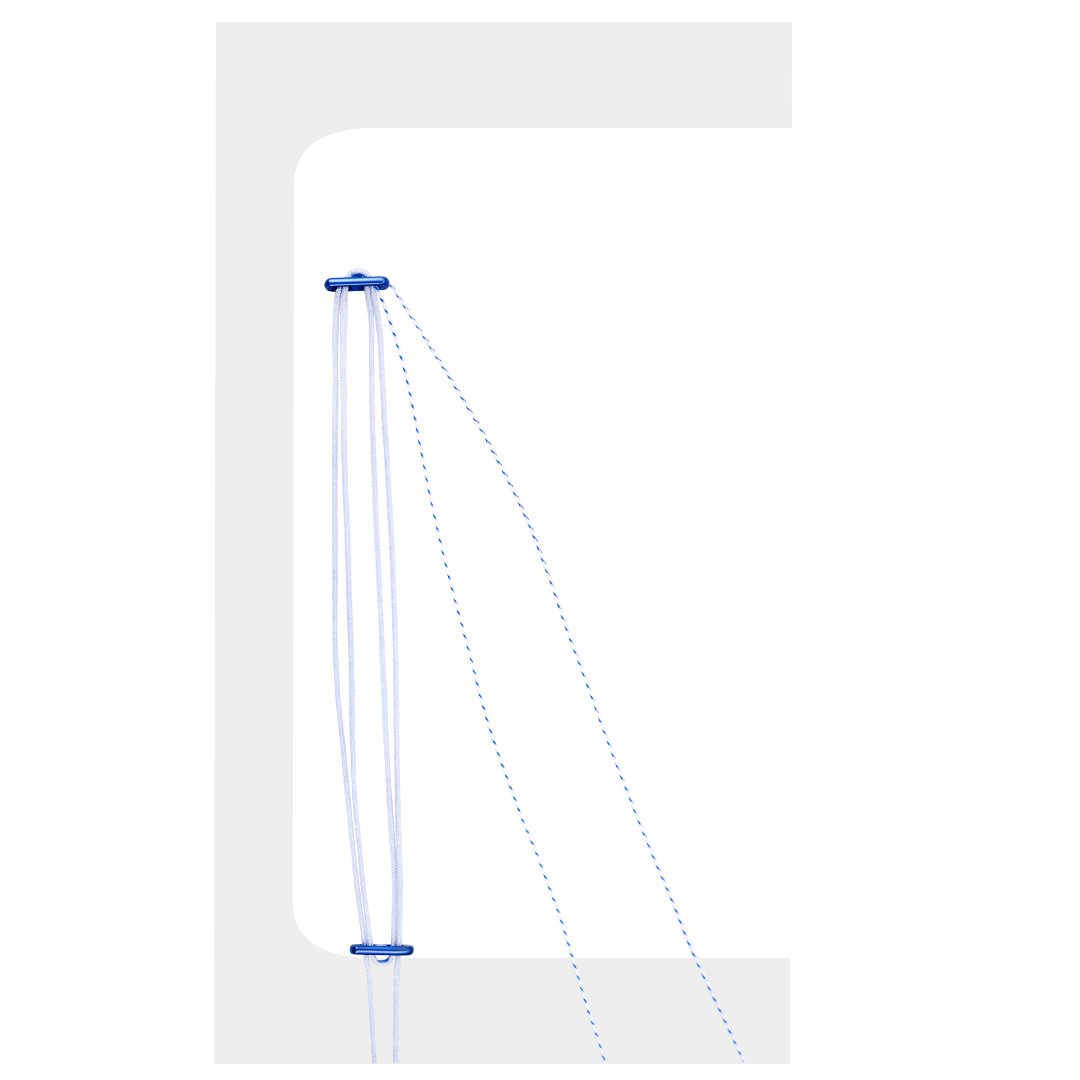
Buttonfix-W two
fixation buttons
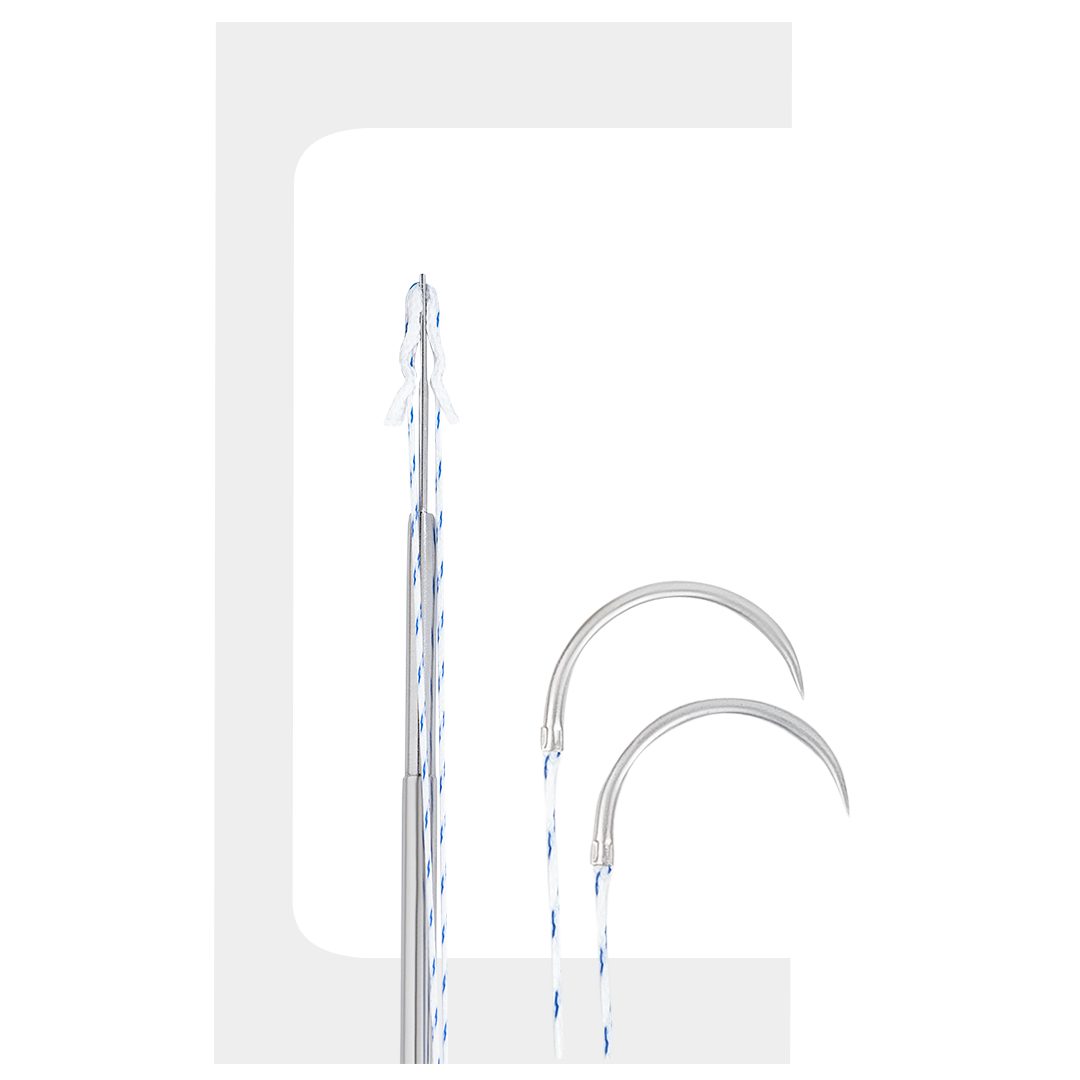
Fiberknot

MICRO-VIM™

MICRO-VIM™ PK
Hip Sports Medicine
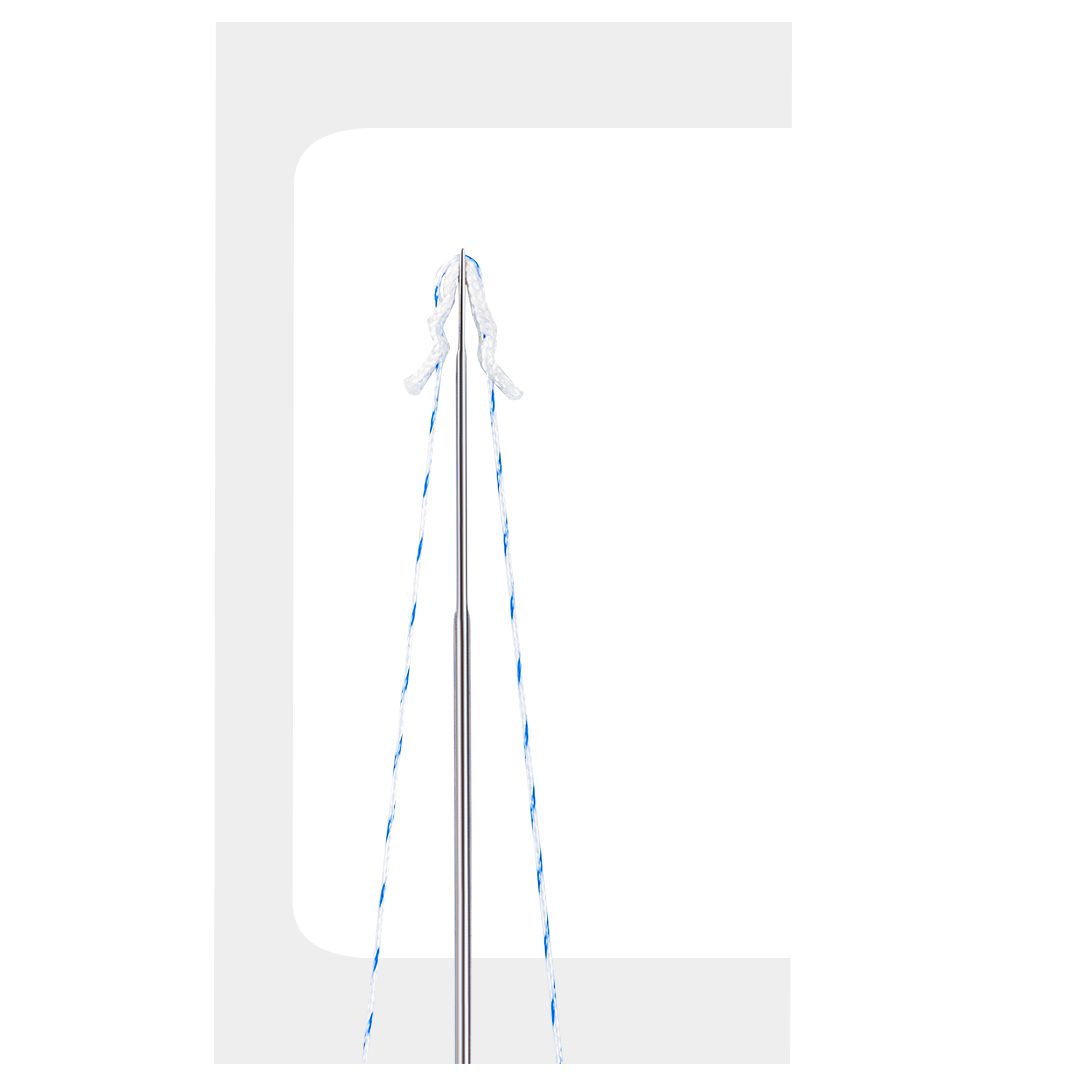
FIBERKNOT®
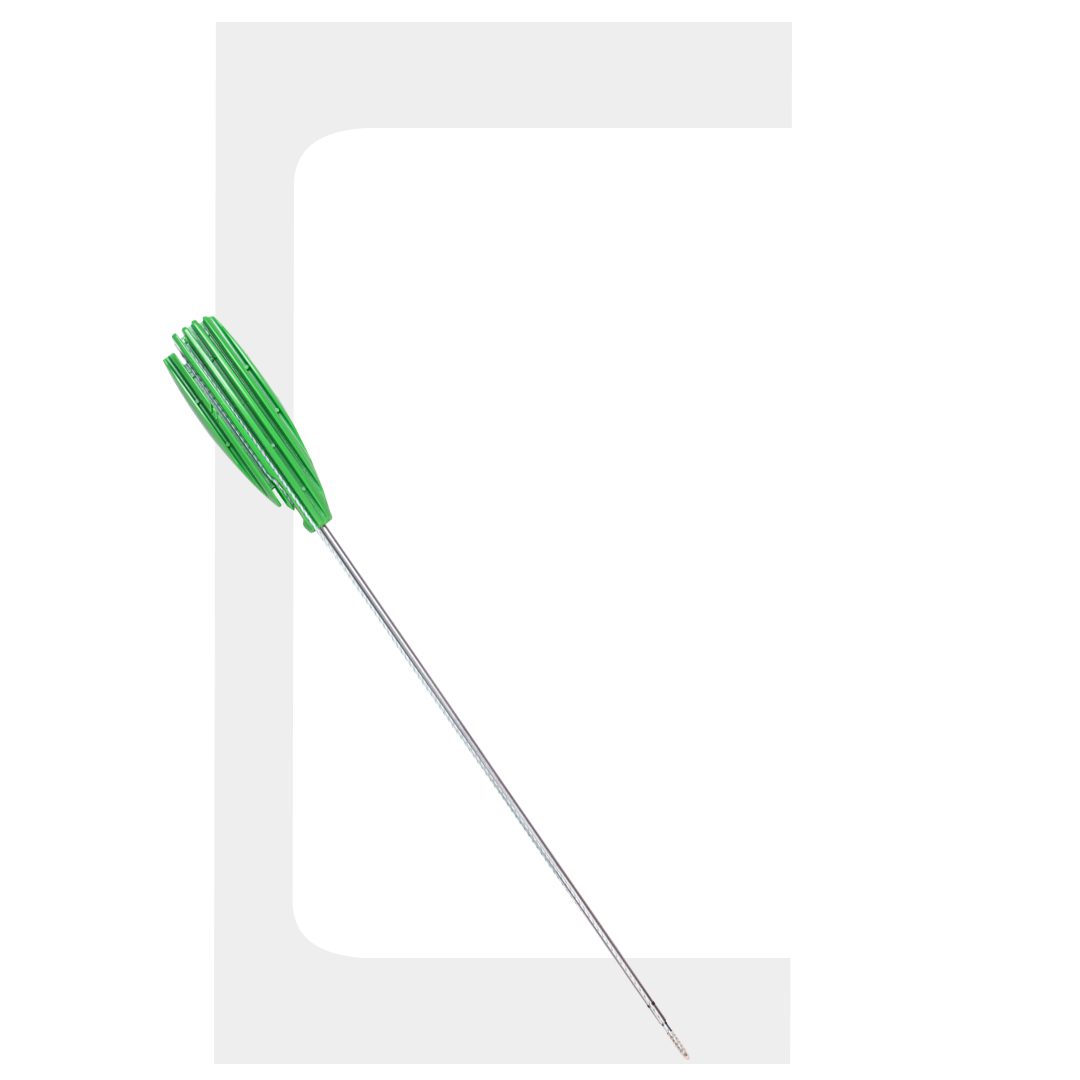
MINI-VIM PK®